Is immune thrombocytopenia to blame?
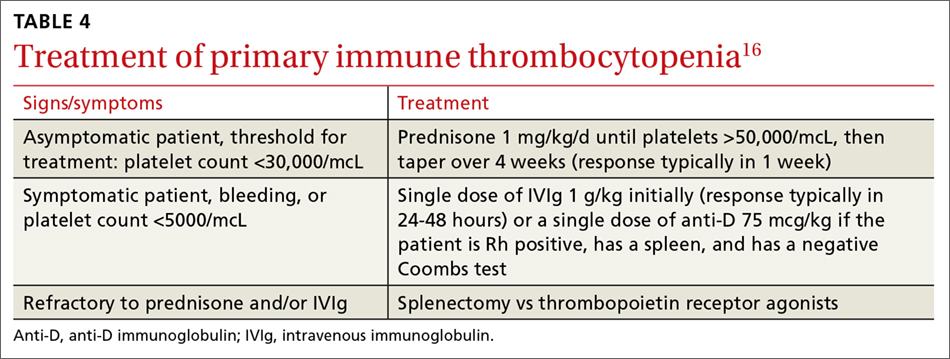
Immune thrombocytopenia (ITP) is an autoimmune disorder resulting in the destruction of normal platelets and may be primary or secondary to processes described previously (HIT, H pylori infection, etc). Consider ITP if, after a thorough work-up, a cause of isolated thrombocytopenia is not identified.16 Treatment for ITP is outlined in TABLE 4.16 FIGURE 1 is an algorithm for the complete evaluation of thrombocytopenia in adults.
Treatment: Platelet transfusions
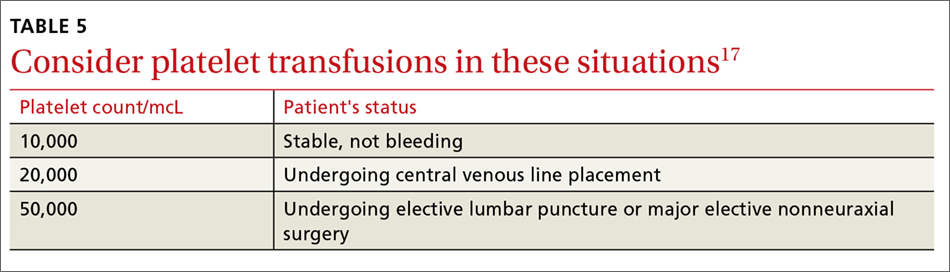
In general, patients who are not actively bleeding are considered stable and do not require platelet transfusions to minimize their risk of bleeding or prevent bleeding during a planned procedure unless their platelet count falls below the levels specified in TABLE 5.17 For patients who are actively bleeding, a more aggressive approach may be required. Locally-derived transfusion protocols typically guide transfusions for the actively hemorrhaging patient. The American Association of Blood Banks has put forth evidence-based guidelines for platelet transfusions when a patient is given a diagnosis of thrombocytopenia (see TABLE 5).17 Single-donor platelets have a shelf life of 3 to 5 days, and one unit will raise platelets 30,000 to 50,000/mcL.
Neutropenia: Prevalence varies by ethnicity
An absolute neutrophil count (ANC) of <1500 cells/mcL traditionally defines neutropenia, with an ANC of 1000 to 1500 cells/mcL constituting mild neutropenia; 500 to 999 cells/mcL, moderate; and <500 cells/mcL, severe.18 Similar to the evaluation of thrombocytopenia, it is important to repeat the CBC prior to initiating a work-up in order to confirm that the neutropenia is not a laboratory error. Additionally, patients with signs or symptoms of infection should be worked up expeditiously.
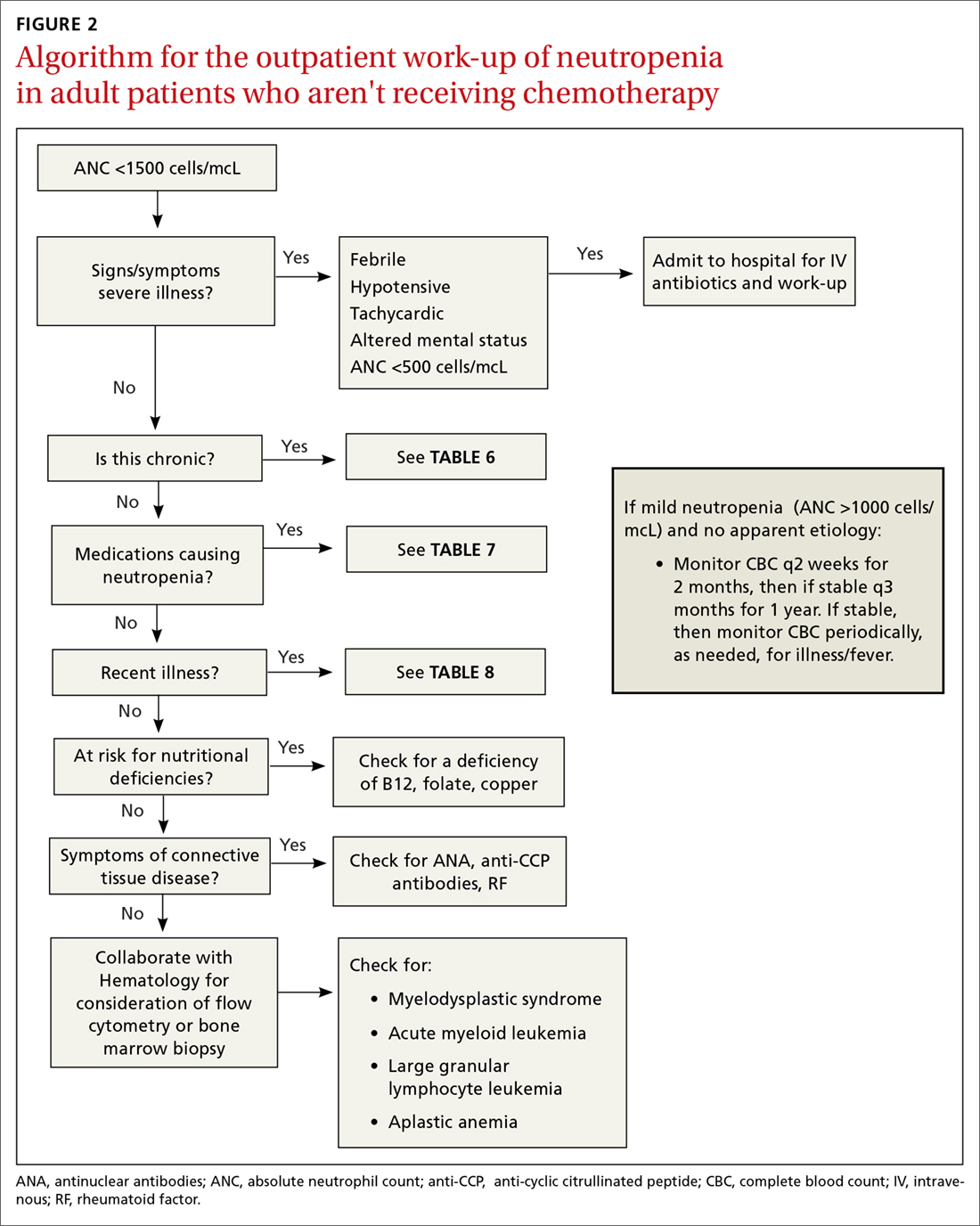
The prevalence of neutropenia varies by ethnicity. According to the National Health and Nutrition Examination Survey 1999 to 2004, the prevalence was 4.5%, 0.79%, and 0.38% in black, white, and Mexican-American participants, respectively.19 FIGURE 2 outlines the outpatient work-up of adult patients with neutropenia not related to chemotherapy.
Continue to: Is the patient severely ill?