
Human beings derive intense pleasure from bubbles and all kinds of foamy products, and scientists have long found them equally fascinating, given the complicated underlying physics. Most recently, a group of Japanese researchers published a paper in Scientific Reports describing two distinct mechanisms by which simple foams collapse. And in a new paper in the Proceedings of the National Academy of Sciences, physicists at MIT and Princeton University demonstrated how to develop spherical bubbles uniformly by confining them in a narrow tube.
Individual bubbles typically form a sphere, because that's the shape with the minimum surface area for any volume and hence is the most energy-efficient. Back in the 19th century, Lord Kelvin proposed a bizarre soccer-ball shape called a tetrakaidecahedron (Greek for "fourteen faces" and sometimes translated "tetradecahedron"), with six square and eight hexagonal faces, to describe a bubble's natural geometry. It's known as "Lord Kelvin's cell," and while it was a valiant effort, that exact structure has yet to be observed in real-world bubbles, although physicists from Trinity College Dublin proposed a better solution to the conundrum in a 1993 paper.
Foams are ubiquitous in everyday life, found in foods (whipped cream), beverages (beer, cappuccino), shaving cream and hair-styling mousse, packing peanuts, building insulation, flame-retardant materials, and so forth. All foams are the result of air being beaten into a liquid formula that contains some kind of surfactant (active surface agent), usually fats or proteins in edible foams, or chemical additives in non-edible products. That surfactant strengthens the liquid film walls of the bubbles to keep them from collapsing.
Ideally, scientists would like to be able to form bubbles with a uniform size and shape, like the liquid droplets that form from a dripping faucet. Both droplets and bubbles start out with the flowing water or air elongating into a neck, then pinching off from the main flow to collapse into spheres. It's what happens every time you dip a bubble-blowing wand into the bubble solution and gently blow through the film that forms along the ring. But droplet and bubble formation are actually inverse physical processes.
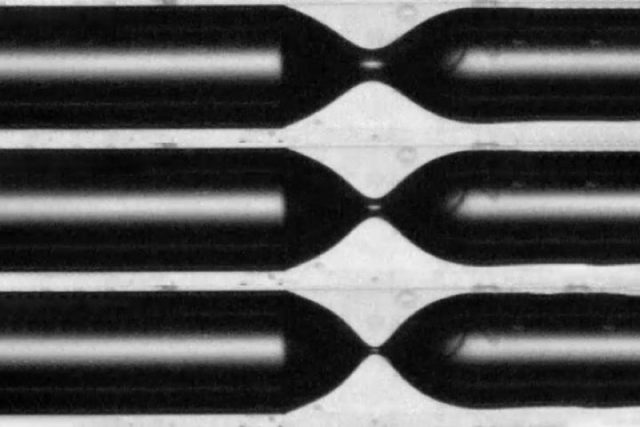
Droplets forming off a dripping faucet is a universal process from a physics standpoint. That means they will be fairly uniform in terms of size and spacing, even if there are differences in initial conditions, such as viscosity of the water, surface tension, or in the size of the faucet opening. However, mix air or gas into a large vat of liquid (like injecting air into a Jacuzzi tub) and bubbles will form in a more random, scattershot fashion, according to Ruben Juanes of MIT, co-author of the PNAS paper.
"If you have irregularities in the orifice, or if the orifice is larger or smaller, or if you inject with some pulsation, all of that will lead to a different pinch-off of the bubbles," Juanes said.
Prof. Juanes and his colleagues at MIT and Princeton conducted experiments involving gas percolating into oil and similar viscous liquids. They found that it was possible to achieve the same kind of universality with bubble formation as with water droplets—at least up to a point—if bubbles formed while confined in a tube. Something about the interactions between the walls of the tube and the bubble makes the whole system less sensitive to irregularities in the initial conditions. That could help control the formation of drops and bubbles in applications such as microfluidics, inkjet printing, or medical imaging.
"Our work is really a tale of two surprising observations; the first surprising observation came around 15 years ago, when another group investigating formation of bubbles in large liquid tanks observed that the pinch-off process is non-universal," said co-author Amir Pahlavan, a graduate student at MIT. He added that the process depends on the details of the experimental setup. "The second surprise now comes in our work, which shows that confining the bubble inside a capillary tube makes the pinch-off insensitive to the details of the experiment and therefore universal."
In a jam
One reason for the minimizing principle when it comes to a bubble's shape is that many bubbles can then tightly pack together to form a foam. But bubbles "coarsen" over time, the result of gravity pulling down on the liquid and thinning out the walls. So eventually they start to look more like soccer balls (polyhedrons). In a coarsening foam, smaller bubbles are gradually absorbed by larger ones. There is less and less liquid to separate the individual bubbles, so they press together to fill the space.
This "jamming" is why foams are typically far more rigid than their gas (95 percent) and liquid (5 percent) components. The more tightly the bubbles jam together, the less they can move around, and the greater the pressure inside them becomes, giving them properties of a solid.
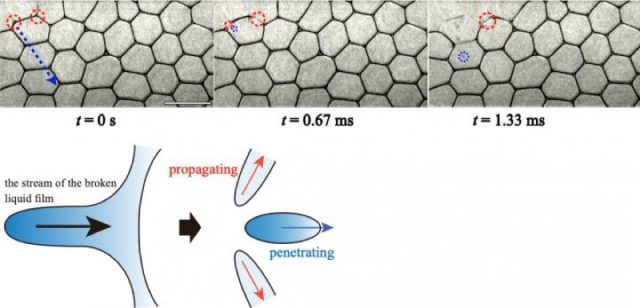
The Japanese researchers from Tokyo Metropolitan University who authored the recent paper in Scientific Reports were particularly interested in studying a phenomenon known as "collective bubble collapse," or CBC, in which breaking one bubble at the edge of a foam results in a cascading effect as the breakage spreads to other bubbles in the foam. The researchers used a typical solution of water, glycerol, and a surfactant to make a simple 2D foam squashed between two plates of glass. Then they painstakingly used a needle to pop a single bubble on the edge of the foam, recording the effects with a high-speed camera.
When they analyzed the data, the scientists identified two distinct mechanisms for the resulting CBCs: a so-called "propagating mode," in which a broken bubble is absorbed into the liquid film, and a "penetrating mode," in which the breakage of a bubble causes droplets to shoot off and hit other bubbles, causing them to break in turn.
Among other useful findings: higher levels of liquid in the foam meant that slower droplets were released in the penetrating mode, slowing the spread of the collapse, since the droplets often lacked sufficient energy to penetrate another bubble's surrounding film. Also, when the researchers changed the viscosity of the fluid, there was no significant impact on how many bubbles broke in the CBC. That's key, because many industrial strategies for stabilizing foams rely on altering the viscosity; this shows those methods are ineffective.
The researchers suggest focusing instead on using several different surfactants in the mixture. This would strengthen the resulting film to make it more resistant to breakage when hit by flying droplets.
DOI: Scientific Reports, 2019. 10.1038/s41598-019-41486-6.
DOI: PNAS, 2019. 10.1073/pnas.1819744116 (About DOIs).
reader comments
23